Leinco Technologies launches novel COVID-19 ImmunoRank™ Neutralization MICRO-ELISA test kit
September 28, 2020
.jpg?width=300&name=COVID-19-ImmunoRank-ELISA-kit%20(1).jpg) The COVID-19 pandemic is one of the most urgent infectious disease challenges the world has faced in recent history. Presently, few treatment options exist, therefore, the need to evaluate neutralizing antibodies to fight the SARS-CoV-2 virus is at an all-time high. Current screening methods to identify circulating SARS-CoV-2 neutralizing antibodies including the plaque reduction neutralization assay and other live virus cell based tests are inefficient and can require multiple days to complete.
The COVID-19 pandemic is one of the most urgent infectious disease challenges the world has faced in recent history. Presently, few treatment options exist, therefore, the need to evaluate neutralizing antibodies to fight the SARS-CoV-2 virus is at an all-time high. Current screening methods to identify circulating SARS-CoV-2 neutralizing antibodies including the plaque reduction neutralization assay and other live virus cell based tests are inefficient and can require multiple days to complete.
As a result and in the pursuit for a better solution, Leinco Technologies has developed a proprietary, fully validated assay termed COVID-19 ImmunoRank™ Neutralization MICRO-ELISA test. This standard 96-well ELISA based assay is a game changer for evaluating convalescent plasma, antibody therapies and determining the effectiveness of vaccines for COVID-19. ImmunoRank™ was developed in collaboration with ADMA Biologics.
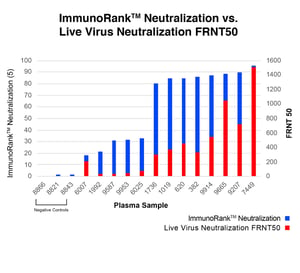
COVID-19 ImmunoRank™ Neutralization MICRO-ELISA
Leinco Technologies, Inc. is a biotechnology company founded in 1992 as a specialty manufacturer of early discovery research products including antibodies, recombinant proteins, ELISA kits, second step reagents and other life sciences products. Shortly thereafter, Leinco also established themselves as a premier provider of custom R&D and manufacturing services focusing on monoclonal antibodies and recombinant proteins. Our innovative products and services are used to augment the early discovery process in life science research, diagnostics and ground breaking development of protein therapeutics. Leinco’s COVID-19 research and development efforts have propelled them forward in providing high purity antibodies and proteins specific to SARS-CoV-2 virus, along with diagnostic kits. Leinco has an extensive product portfolio of SARS-CoV-2 antibodies, proteins, and assay development reagents. Leinco operates in a manufacturing facility located in St. Louis, Missouri. Leinco is an ISO9001.2015 certified company and all manufacturing data is recorded in cGMP compliant batch records.
About ADMA Biologics, Inc.
ADMA Biologics is an end-to-end commercial biopharmaceutical company dedicated to manufacturing, marketing and developing specialty plasma-derived biologics for the treatment of immunodeficient patients at risk for infection and others at risk for certain infectious diseases. ADMA currently manufactures and markets three United States Food and Drug Administration (FDA) approved plasma-derived biologics for the treatment of immune deficiencies and the prevention of certain infectious diseases: ASCENIV™ (immune globulin intravenous, human – slra 10% liquid) for the treatment of primary humoral immunodeficiency (PI); BIVIGAM® (immune globulin intravenous, human) for the treatment of PI; and NABI-HB® (hepatitis B immune globulin, human) to provide enhanced immunity against the hepatitis B virus. ADMA manufactures its immune globulin products at its FDA-licensed plasma fractionation and purification facility located in Boca Raton, Florida. Through its ADMA Bio Centers subsidiary, ADMA also operates as an FDA-approved source plasma collector in the U.S., which provides a portion of its blood plasma for the manufacture of its products. ADMA’s mission is to manufacture, market and develop specialty plasma-derived, human immune globulins targeted to niche patient populations for the treatment and prevention of certain infectious diseases and management of immune compromised patient populations who suffer from an underlying immune deficiency, or who may be immune compromised for other medical reasons. ADMA has received U.S. Patents: 9,107,906, 9,714,283, 9,815,886, 9,969,793 and 10,259,865 related to certain aspects of its products and product candidates. For more information, please visit www.admabiologics.com





